Heterogeneous Fischer-Tropsch Synthesis (FTS) Reactions, ANSYS Fluent Simulation
$260.00 Student Discount
- This report presents the simulation of a reactive multiphase system incorporating heterogeneous Fischer-Tropsch synthesis reactions in a porous medium
- An iron (Fe) catalyst catalyzes the reactions, and the reaction rates are determined using Arrhenius kinetics.
- The Eulerian multiphase model is employed to investigate the interaction between different phases
- A Lee mass transfer model is applied for the phase change of water between liquid and gaseous states.
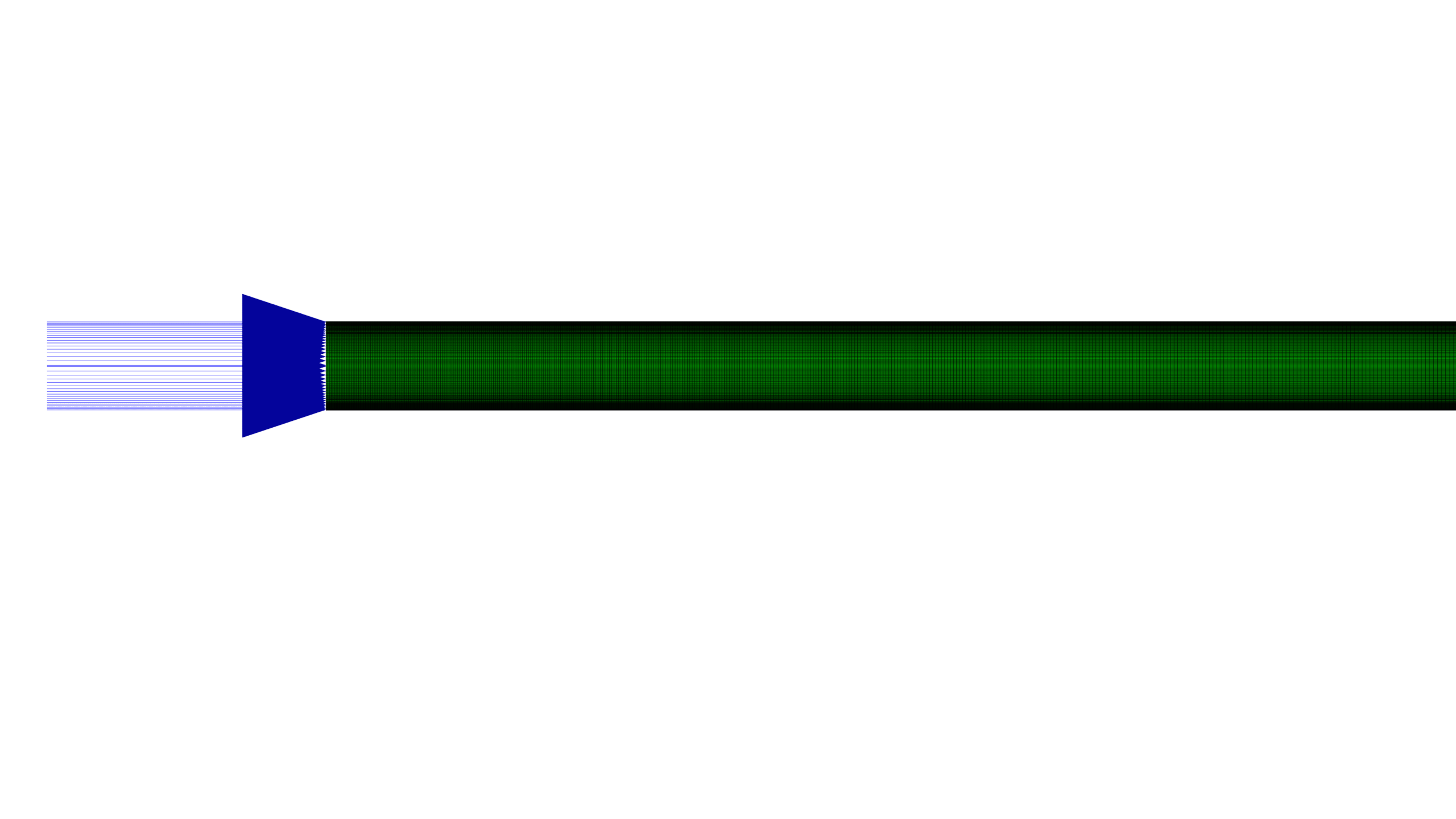
- A 2D geometry was created using SpaceClaim and The domain was meshed in ANSYS Meshing, using a structured mesh with 105,000 structured elements.
To Order Your Project or benefit from a CFD consultation, contact our experts via email (info@mr-cfd.com), online support tab, or WhatsApp at +44 7443 197273.
There are some Free Products to check our service quality.
If you want the training video in another language instead of English, ask it via info@mr-cfd.com after you buy the product.
Description
Simulation of Multiphase and Heterogeneous Fischer-Tropsch synthesis (FTS) Reactions in a Porous Medium Using the Eulerian Model
Description
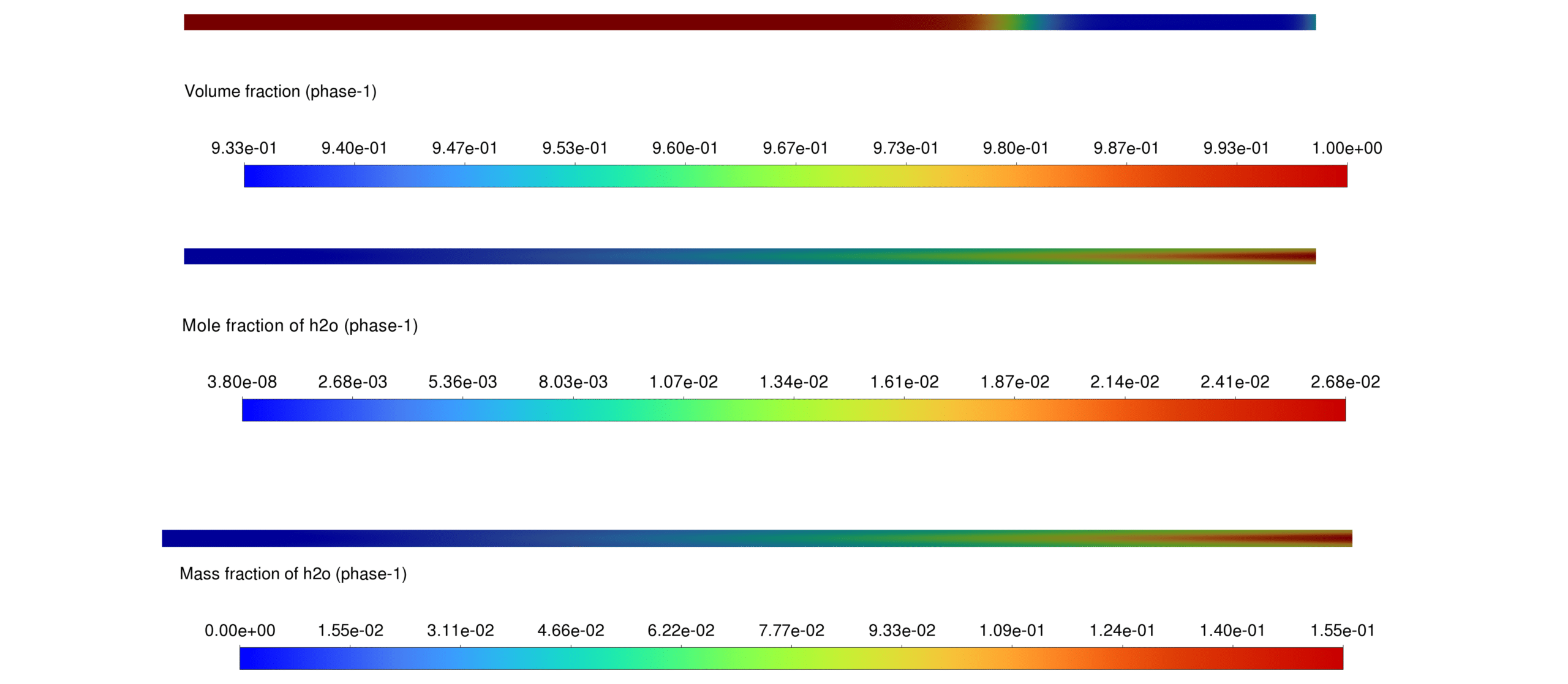
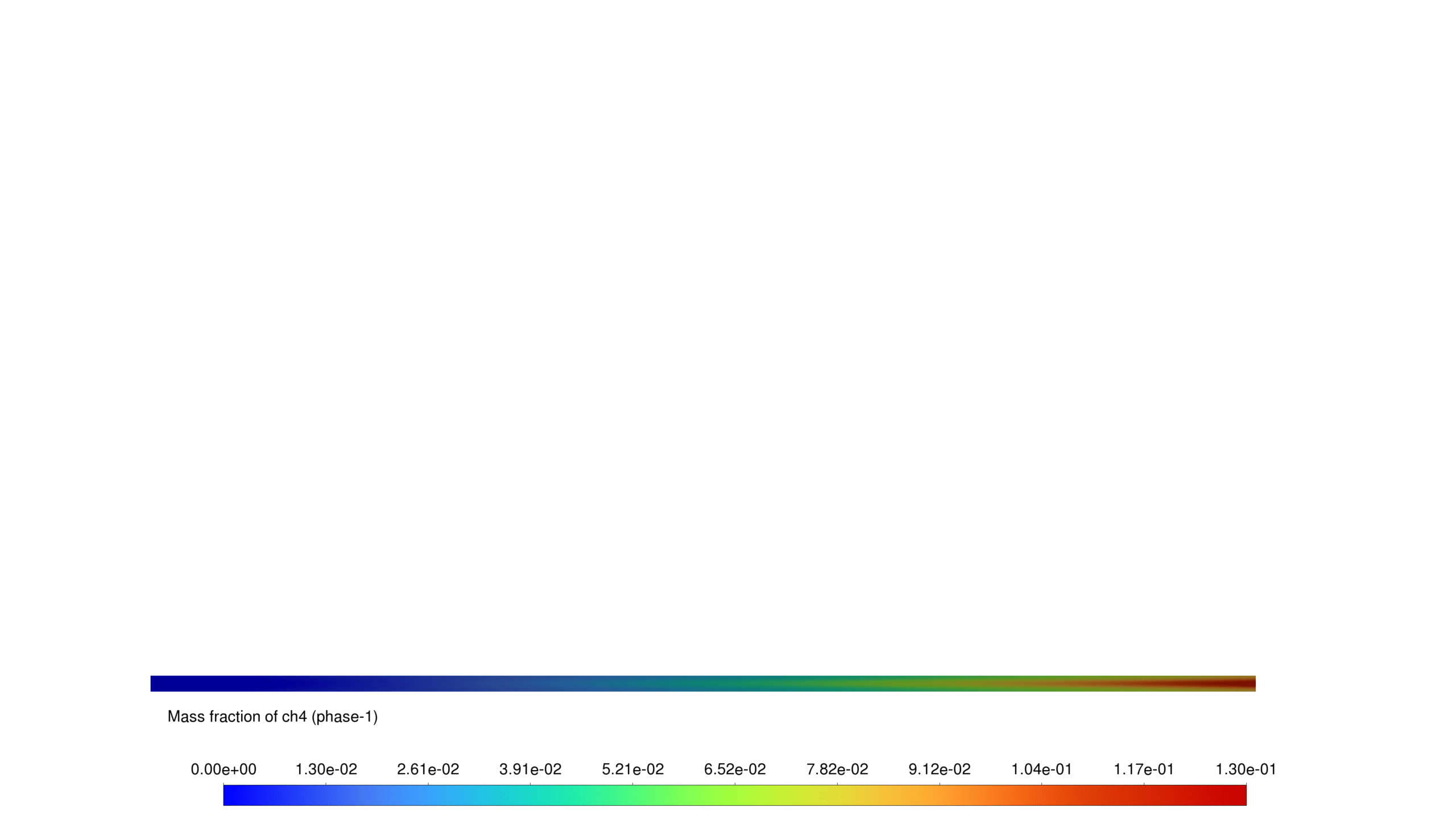
This report presents the simulation of a reactive multiphase system incorporating heterogeneous reactions in a porous medium. The Eulerian multiphase model is employed to investigate the interaction between different phases, specifically focusing on water phase transitions and the production of hydrocarbons (CH₄, C₂H₄, C₃H₆, and C₄H₁₀).
A Lee mass transfer model is applied for the phase change of water between liquid and gaseous states, ensuring accurate representation of the vaporization-condensation processes. Using a porous medium in the simulation accounts for an iron (Fe) catalyst, essential for facilitating Fischer-Tropsch reactions.
Fischer-Tropsch synthesis (FTS) is a set of catalytic chemical reactions that convert a mixture of carbon monoxide (CO) and hydrogen (H₂) into hydrocarbons. The general response for Fischer-Tropsch synthesis is:
(2n+1)H2+nCO→CnH2n+nH2O
The reactions considered in this simulation include:
- CO + 3H₂ → CH₄ + H₂O
- 2CO + 4H₂ → C₂H₄ + 2H₂O
- 3CO + 6H₂ → C₃H₆ + 3H₂O
- 4CO + 10H₂ → C₄H₁₀ + 4H₂O
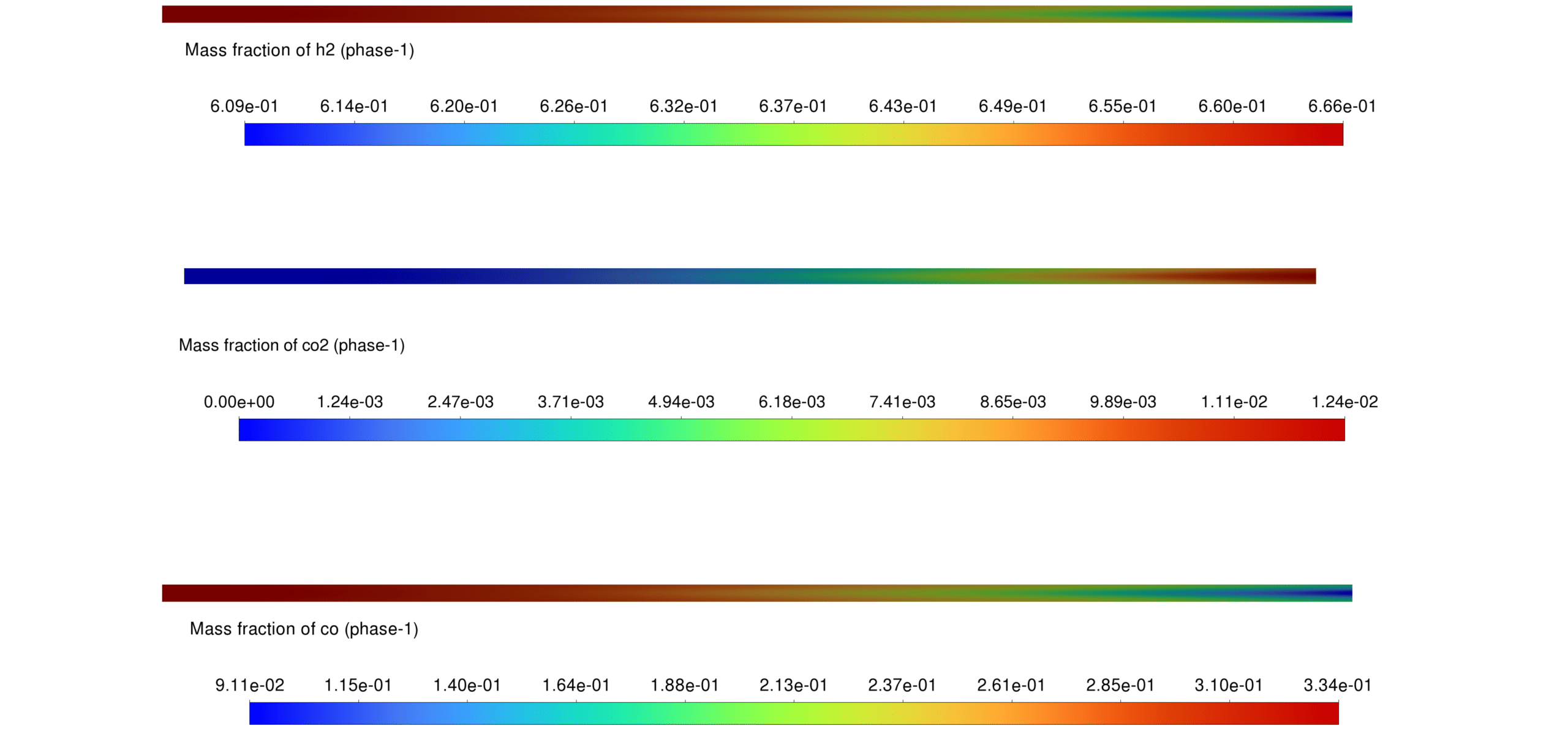
- CO + H₂O → CO₂ + H₂
An iron (Fe) catalyst catalyzes the reactions, and the reaction rates are determined using Arrhenius kinetics specific to Fe-based Fischer-Tropsch synthesis.
The simulation was conducted using ANSYS Fluent, A 2D geometry was created using SpaceClaim and The domain was meshed in ANSYS Meshing, using a structured mesh with 105,000 structured elements.
Methodology
- A horizontal channel of varying length (7 m), width (0.05 cm), with symmetry boundary condition.
- The catalyst bed is a porous medium that accounts for the presence of the Fe catalyst, which influences the reaction kinetics.
- The Eulerian multiphase model is employed to investigate the interaction between different phases.
- A Lee mass transfer model is applied for the phase change of water between liquid and gaseous states.
Results
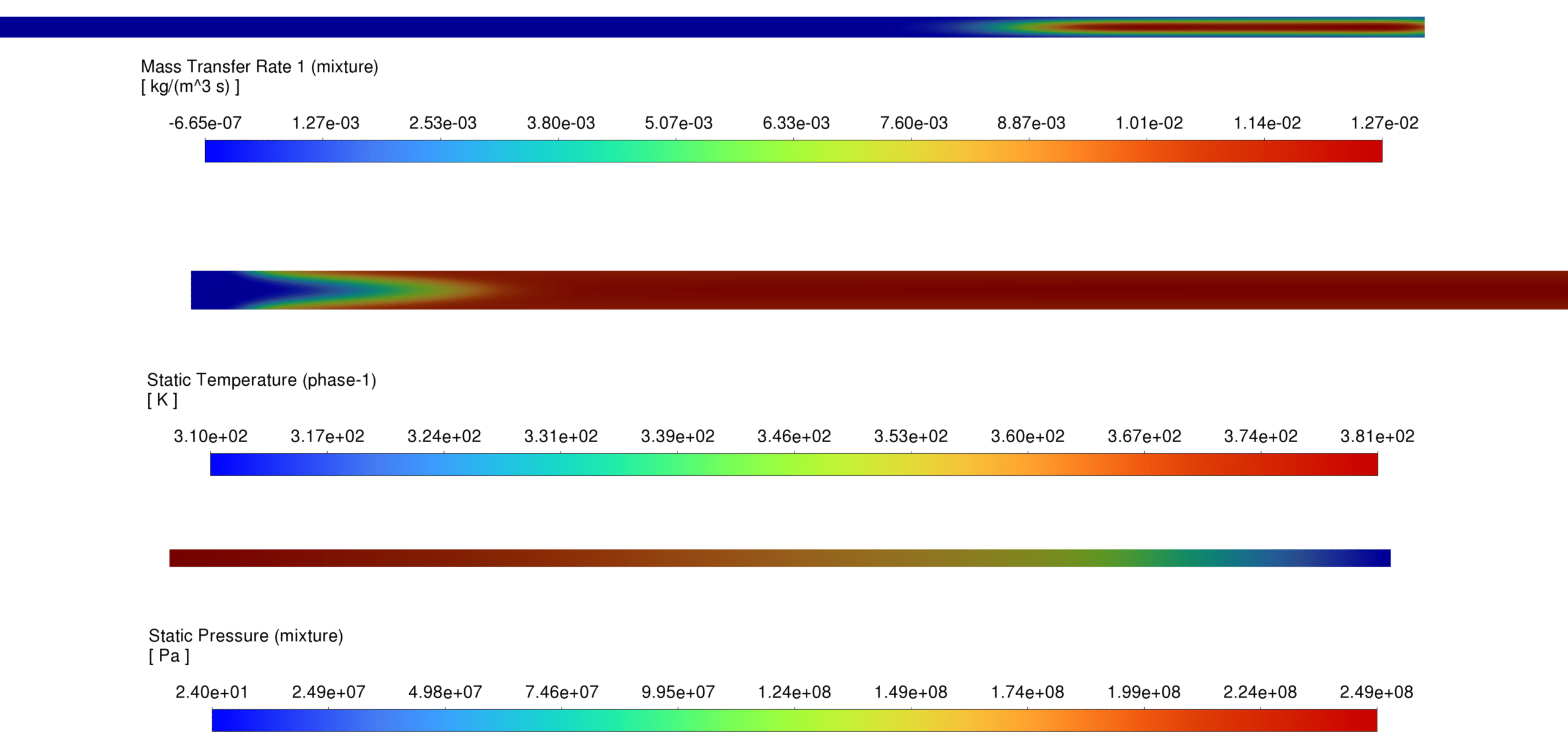
Pressure Distribution The pressure field indicates stable flow development with minor pressure drops near reaction zones due to local expansion effects from phase changes and gas formation.
Temperature Distribution The temperature field demonstrates localized heating near reaction sites, with peak temperatures observed in regions with intense CH₄ and C₂H₄ formation.
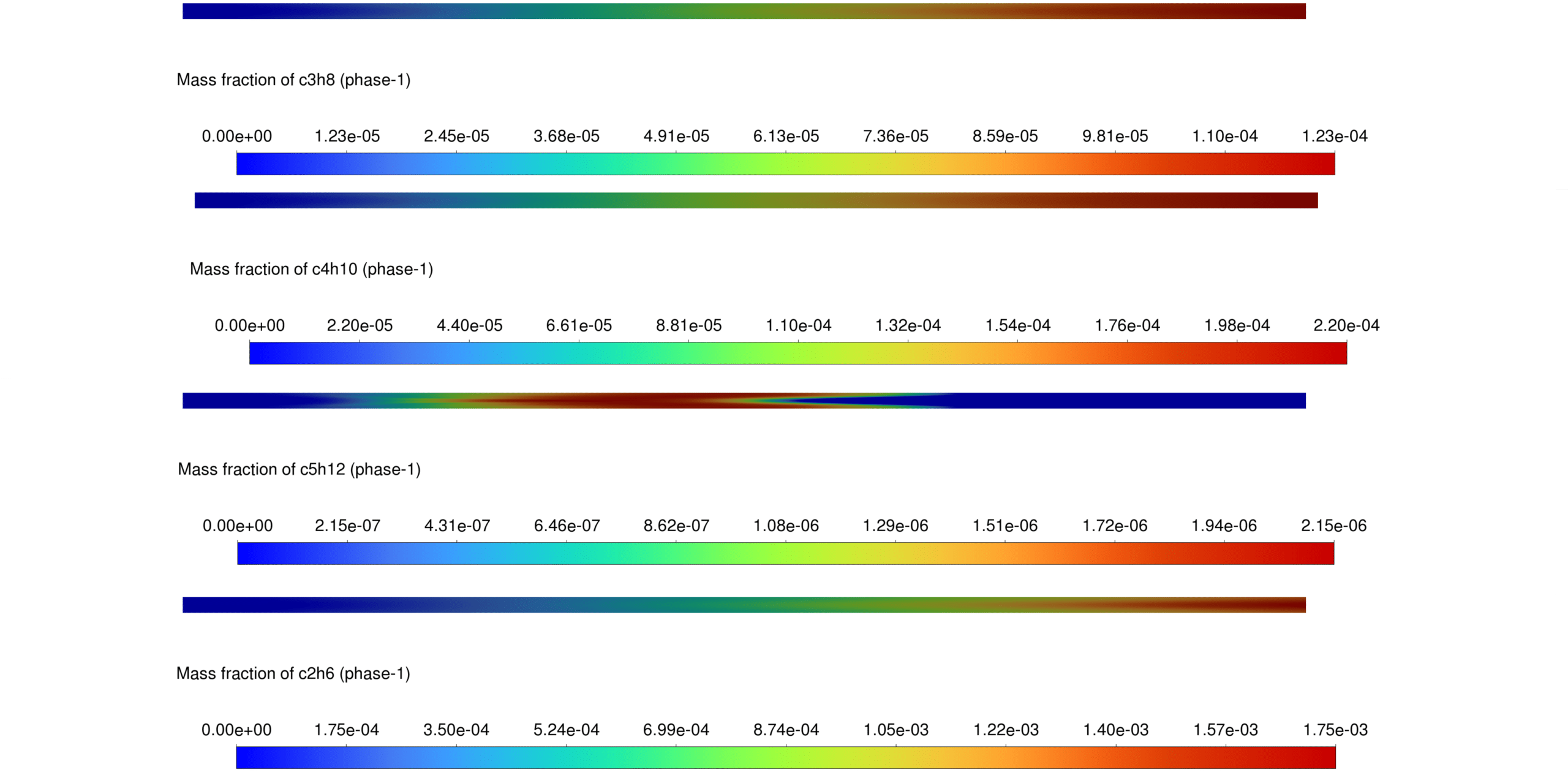
Mass Concentration of Species
- CH₄ shows a gradual increase downstream.
- C₂H₄ and C₄H₁₀ exhibit spatial dependence, with higher concentrations near liquid water presence.
- CO₂ distribution follows combustion reaction trends.
Phase Fraction Analysis The phase fraction distribution illustrates the conversion of H₂O(l) to H₂O(g), validating the phase transition model.
Mass Transfer Rate The Lee model predicts the expected mass transfer rates, with a higher vaporization rate near heat sources and reaction zones.
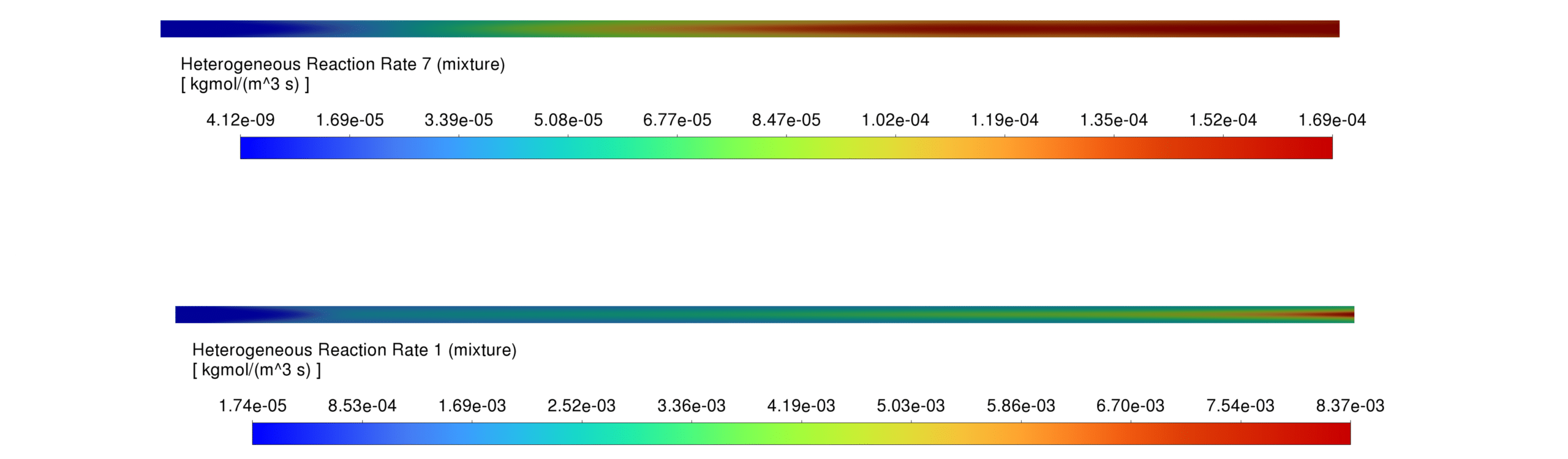
Reaction Rates Reaction rates are highest in regions with both liquid and gaseous water phases, enhancing hydrocarbon production. Applying Arrhenius kinetics for Fe-catalyzed Fischer-Tropsch reactions ensures accurate prediction of hydrocarbon formation.


Reviews
There are no reviews yet.