Alkaline Electrolysis, CFD Simulation, ANSYS Fluent
$330.00 $165.00 Student Discount
- The problem numerically simulates the Alkaline Electrolysis using ANSYS Fluent software.
- We design the 3-D model with the Design Modeler software.
- We mesh the model with ANSYS Meshing software.
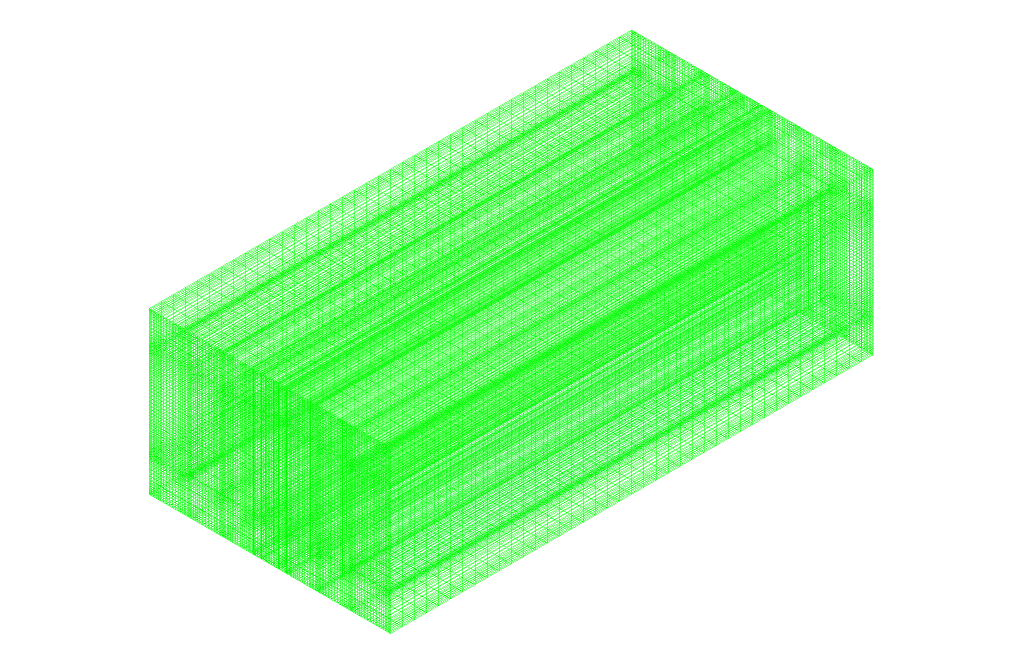
- The mesh is Structured, and the element number equals 428,800.
- We used the Potential/Electrochemistry model to define the potential equation.
- We used the Electrolysis sub-model and Alkaline Electrolysis type to define the electrolysis process.
- We used the Species model to define H2, O2, and H2O.
- We used the Mixture Multiphase model to define water.
To Order Your Project or benefit from a CFD consultation, contact our experts via email (info@mr-cfd.com), online support tab, or WhatsApp at +44 7443 197273.
There are some Free Products to check our service quality.
If you want the training video in another language instead of English, ask it via info@mr-cfd.com after you buy the product.
Description
Description
In this project, we perform the numerical simulation of the Alkaline Electrolysis using ANSYS Fluent software.
This product is the fourth chapter of the Electrolysis Training Course.
Note that a Fuel Cell is an energy conversion device that converts the chemical energy of fuel into electrical energy. Electrolysis is the reverse of a fuel cell. It means power (electricity) is consumed to produce fuel (hydrogen).
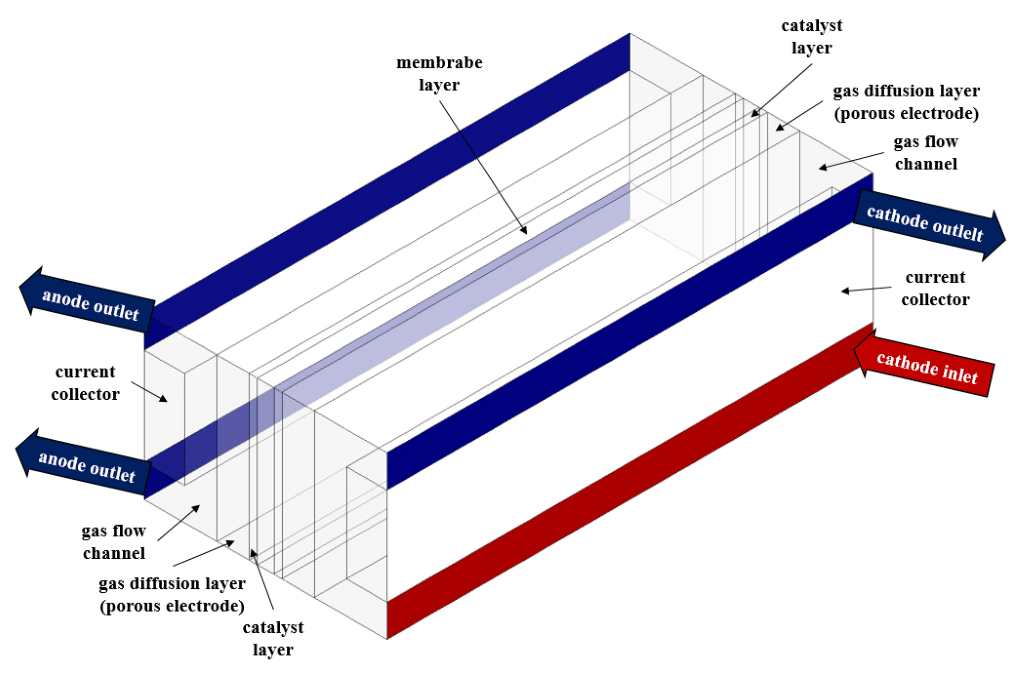
Similar to fuel cells, electrolyzers consist of two main sides called anode and cathode, which are connected by an electrolyte membrane layer. On each side of the anode or cathode, there is a current collector, a gas diffusion layer (porous electrode), and a catalyst layer.
Electrochemical reactions take place in catalyst layers. As a result of these reactions, water is consumed and oxygen and hydrogen are produced.
There are several types of electrolysis processes. In this CFD simulation, we investigated an Alkaline electrolyzer. In Alkaline electrolysis, water is supplied on the cathode side; as a result, hydrogen is produced on the cathode side and oxygen is produced on the anode side.
We performed the simulation process in ANSYS Fluent software.
First, we modeled the geometry in 3D using Design Modeler software. The computational domain is related to the interior space of an electrolyzer.
Then, we meshed the model using ANYS Meshing software. The meshing is structured, and 428,800 cells are generated.
Electrolysis Methodology
We simulated this project based on the CFD method by ANSYS Fluent software.
In this CFD simulation, we used the potential/electrochemistry model to define the potential equation. Then, we used the electrolysis sub-model to define the electrolysis process. In the electrolysis submodel, we selected the Alkaline electrolysis type.
In the electrolysis model setting, we determine the different layers of the electrolyzer. The electrolyzer consists of the cathode, anode, and electrolyte layers. Each of the anode and cathode sides consists of current collectors, gas diffusion layers (porous electrode layers), catalyst layers, and gas flow channels.
Since we used the electrolysis model, the species model and Multiphase model are automatically activated.
First, we used the mixture multiphase model to define a two-phase flow including water and gaseous species. Then, we used the species transport model to define the species present in the electrochemical reactions, including H2, O2, and H2O.
Conclusion
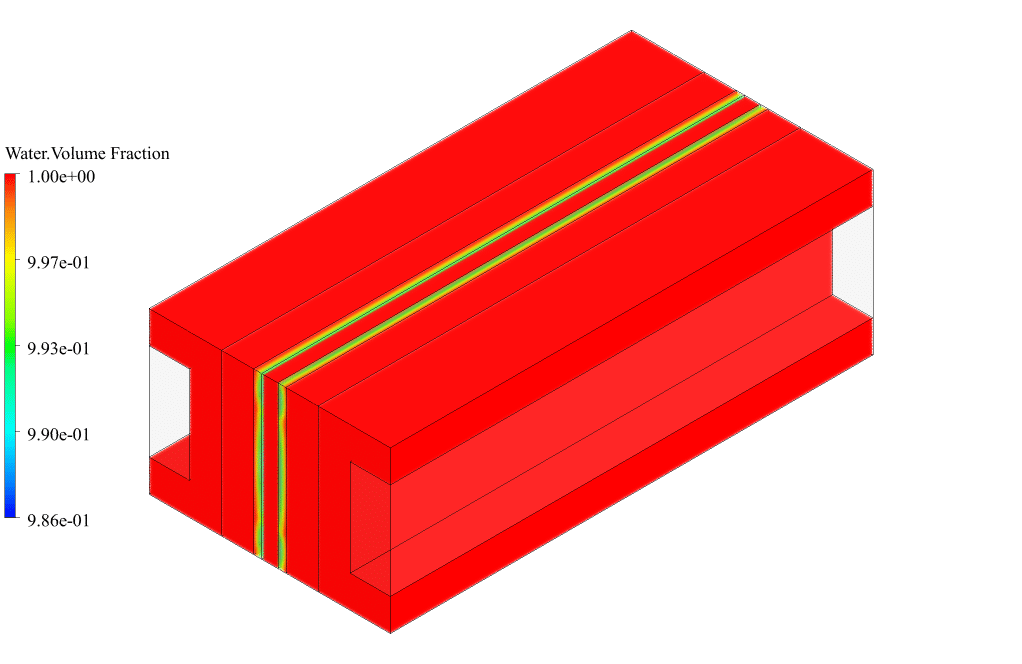
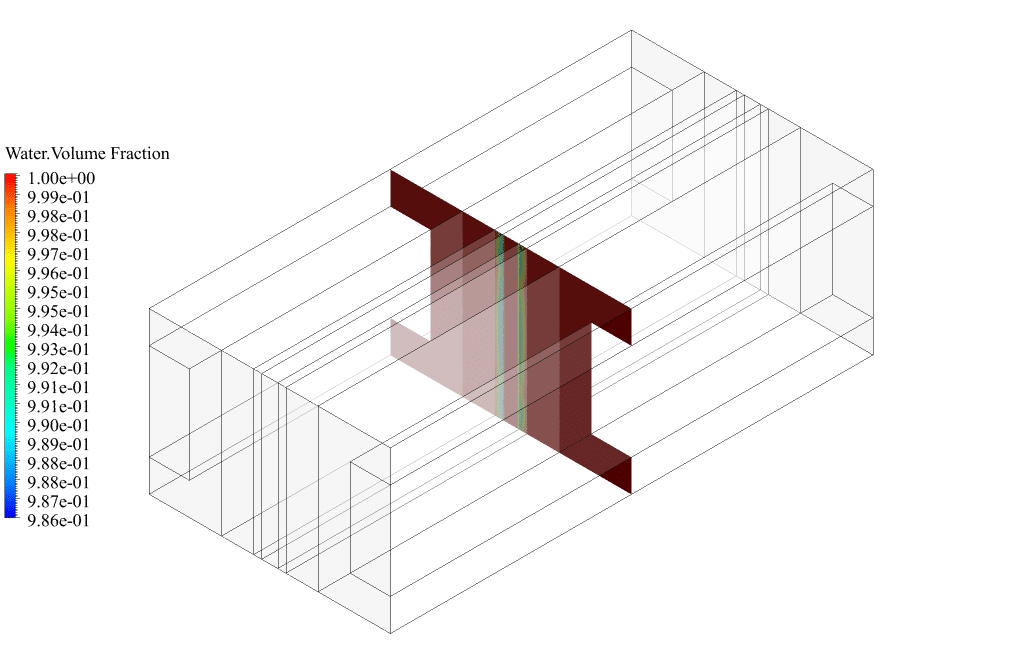
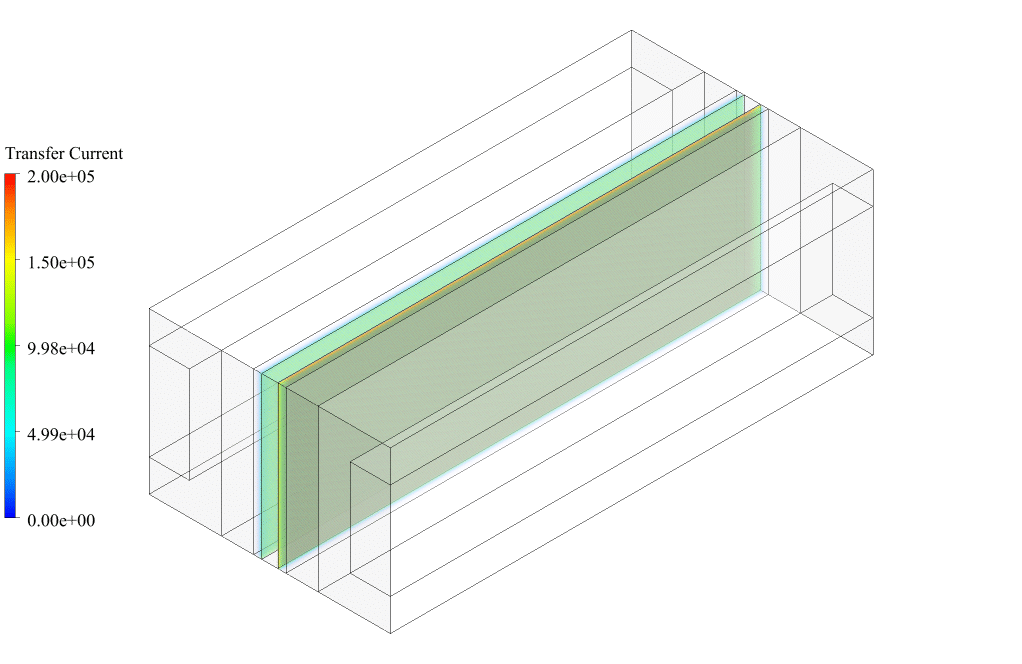
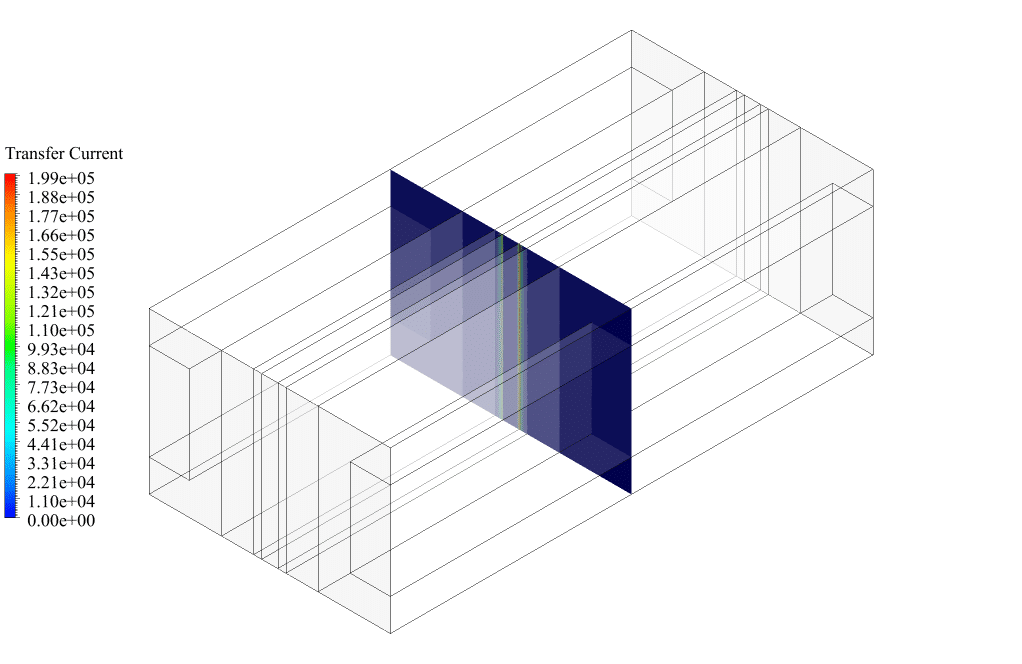
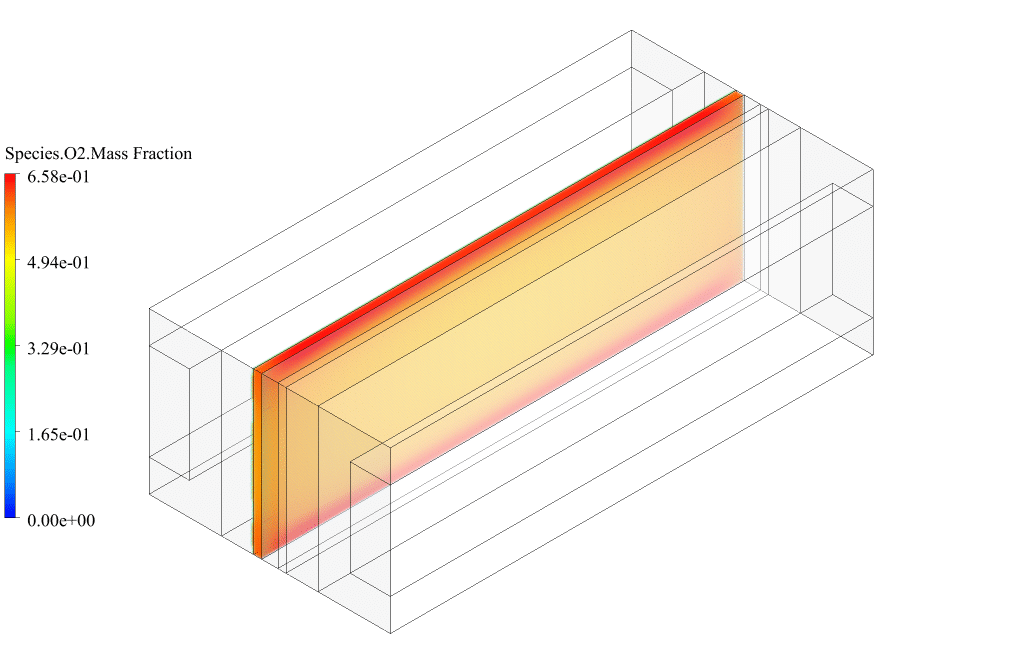
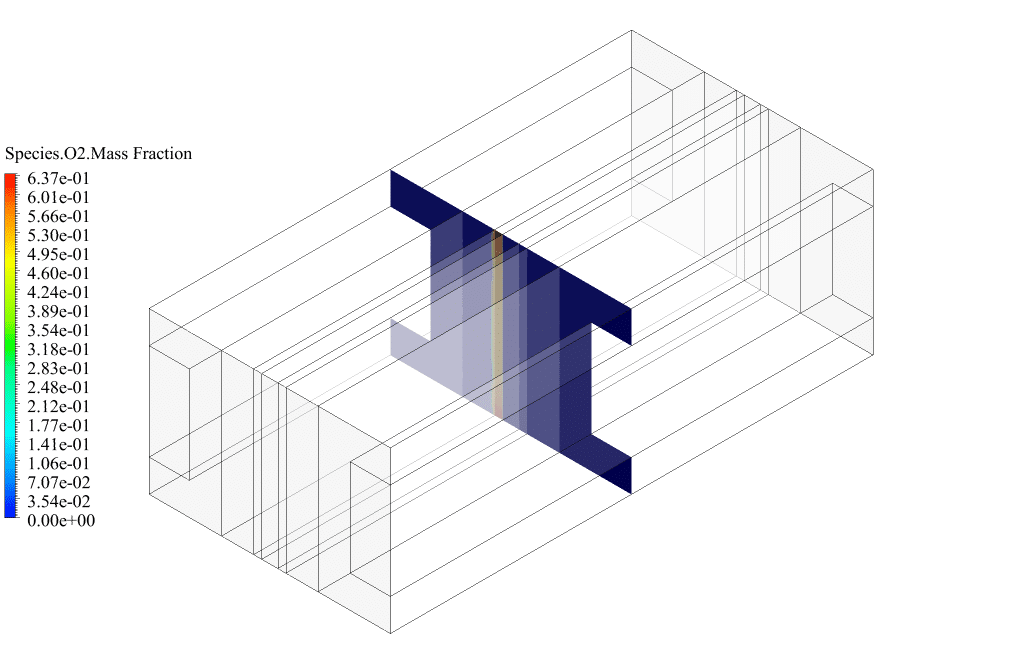
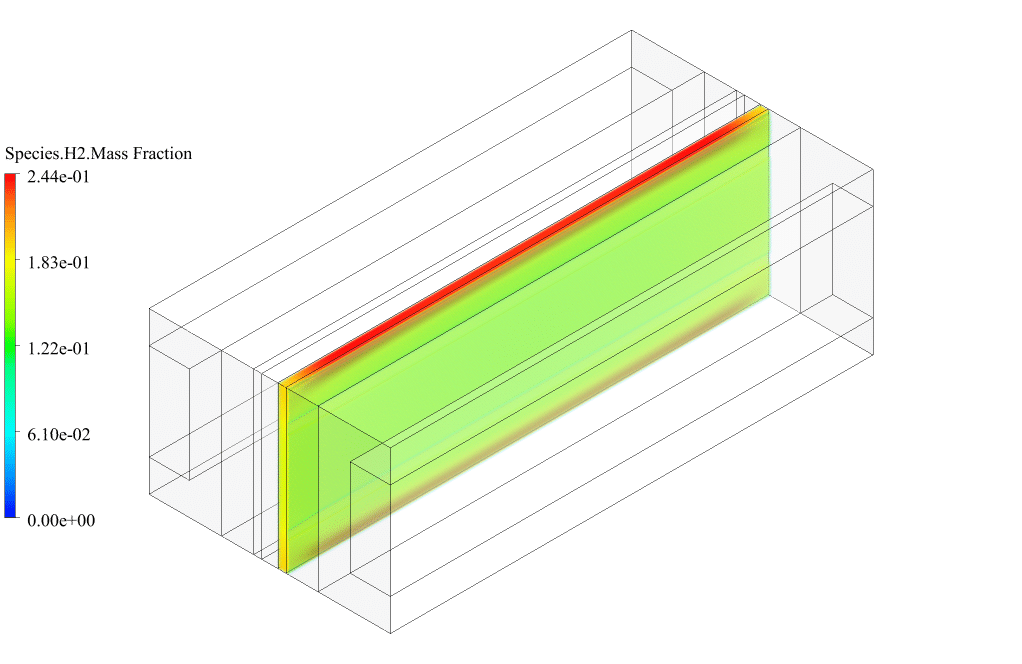
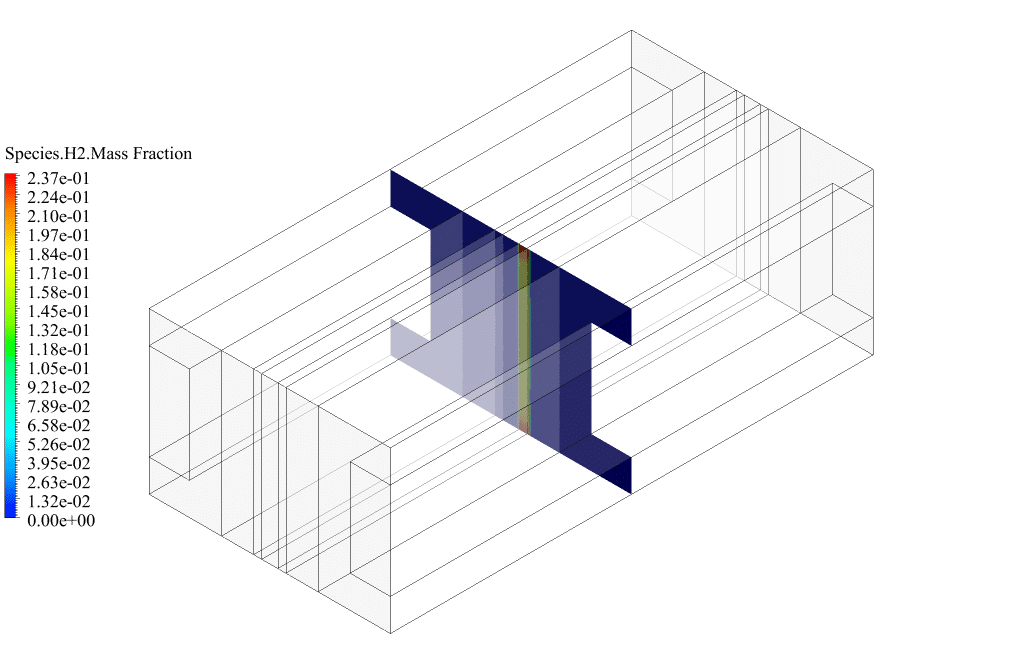
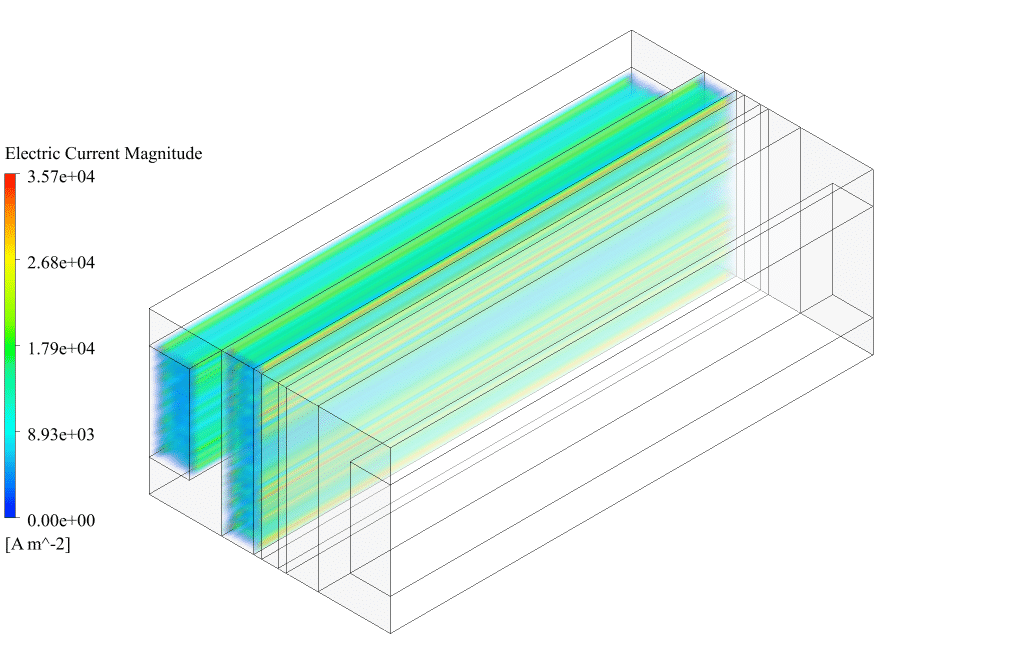
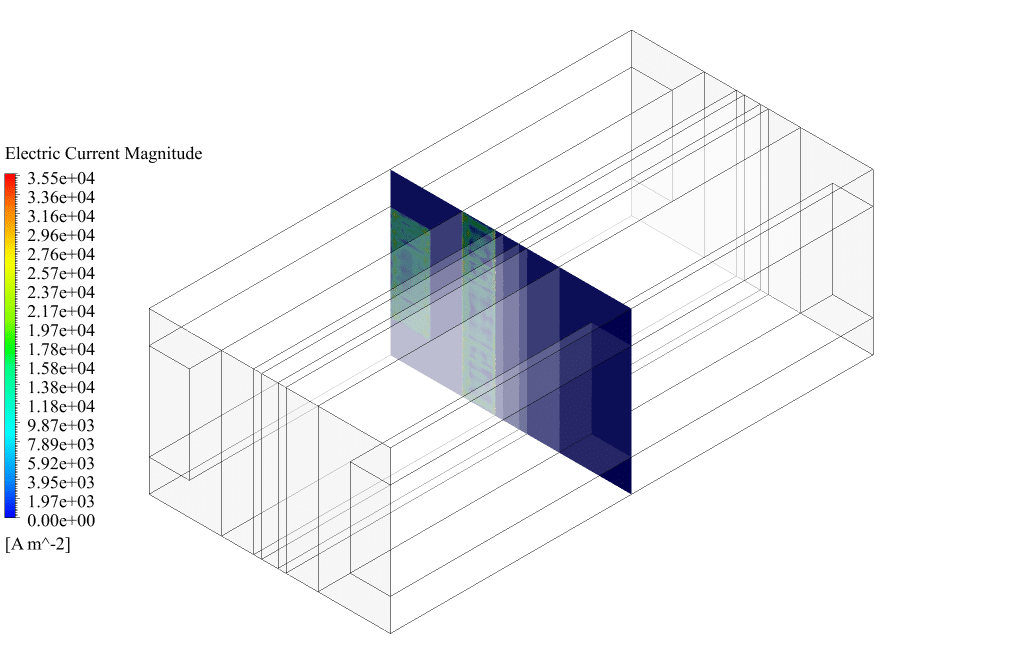
After conclusion, we have obtained the contours related to current flux density magnitude, transfer current, volume fraction of water, and mass fraction of H2 and O2.
The contours of the water volume fraction and H2 and O2 mass fraction show that water enters from the cathode side, hydrogen is produced on the cathode side, and oxygen is produced on the anode side. This behavior is exactly according to the operating mechanism of the Alkaline electrolysis system.
Therefore, we conclude that we performed the electrolyzer simulation correctly.


Reviews
There are no reviews yet.