Catalytic Combustion of Methane in a Micro-Pipe Reactor: Applying CHEMKIN File
$180.00 $90.00 Student Discount
- This report presents a simulation which investigates the catalytic combustion of methane in a micro-pipe reactor.
- A CHEMKIN file was created to define detailed wall surface reactions based on the reactions reported by Deutschmann (1996).
- The geometry of the micro-reactor was created using ANSYS SpaceClaim software.
- A high-quality hexahedral elements mesh was generated using ANSYS Meshing software.
- Appropriate species transport settings and boundary conditions were applied to correctly simulate the volumetric and wall surface reactions.
To Order Your Project or benefit from a CFD consultation, contact our experts via email (info@mr-cfd.com), online support tab, or WhatsApp at +44 7443 197273.
There are some Free Products to check our service quality.
If you want the training video in another language instead of English, ask it via info@mr-cfd.com after you buy the product.
Description
Combustion of Methane in the Presence of Platinum Catalyst: Defining CHEMKIN File for Both Volumetric and Surface Wall Reaction by ANSYS Fluent
Catalytic combustion of methane represents a significant avenue for clean energy production and emissions reduction through controlled oxidation processes. This study investigates the complex interplay between homogeneous gas-phase (volumetric) reactions and heterogeneous surface reactions occurring on platinum catalyst surfaces within a micro-pipe reactor configuration. Understanding these multi-phase reaction mechanisms is crucial for designing efficient catalytic systems for applications ranging from power generation to chemical synthesis and emissions control.
The study highlights the implementation of detailed surface reaction kinetics through custom-developed CHEMKIN files, which define the complex adsorption, desorption, and catalytic conversion processes occurring at the platinum-gas interface. This approach enables precise tracking of surface coverage dynamics, particularly for key species such as Pt, while simultaneously resolving the gas-phase reaction progression throughout the micro-pipe domain. The simulation framework provides valuable insights into the interrelationship between flow characteristics, heat transfer phenomena, and multi-phase chemical reactions that govern catalytic combustion efficiency in micro-scale reactor configurations.
The reaction mechanisms considered in this study include the following:
Volumetric Reactions: (Some reactions have a rate exponent)
CH4 + 1.5O2 => CO + 2H2O
CO + 0.5O2 => CO2
CO2 => CO + 0.5O2
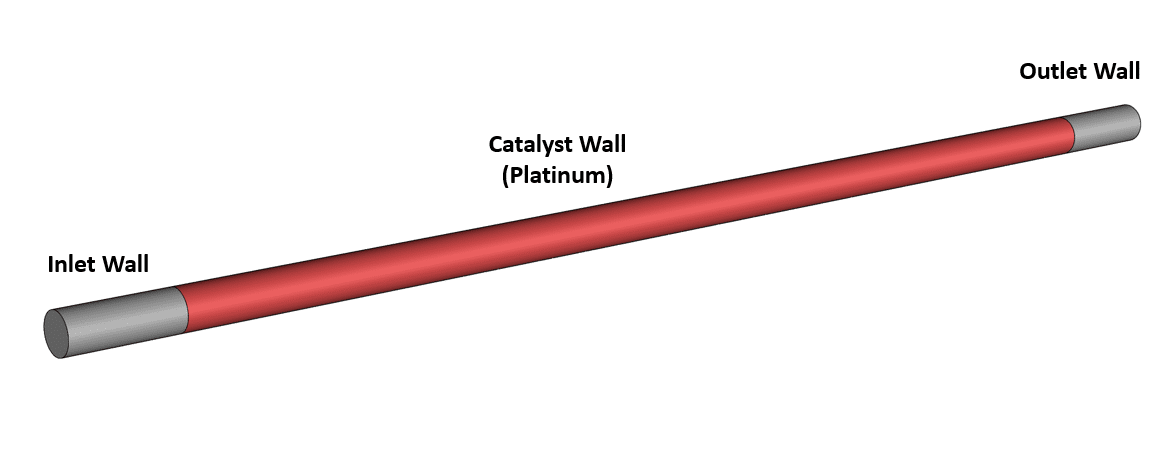
Wall Surface Reactions: (Mechanisms from Olaf Deutschmann Dissertation, 1996)
H2(S) + 2PT(S) => 2H(S) 2H(S) => H2 + 2PT(S)
O2 + 2PT(S) => 2O(S) O2 + 2PT(S) => 2O(S)
2O(S) => O2 + 2PT(S) H2O + PT(S) => H2O(S)
H2O(S) => H2O + PT(S) OH + PT(S) => OH(S)
OH => OH + PT(S) H(S) + O(S) = OH(S) + PT(S)
H(S) + OH(S) = H2O(S) + PT(S) OH(S) + OH(S) = H2O(S) + O(S)
CO + PT(S) => CO(S) CO(S) => CO + PT(S)
CO2(S) => CO2 + PT(S) CO(S) + O(S) => CO2(S) + PT(S)
CH4 + 2PT(S) => CH3(S) + H(S) CH3(S) + PT(S) => CH2(S) + H(S)
CH2(S) + PT(S) => CH(S) + H(S) CH(S) + PT(S) => C(S) + H(S)
C(S) + O(S) => CO(S) + PT(S) CO(S) + PT(S) => C(S) + O(S)
The reactor geometry consists of a straight 3-dimensional cylindrical pipe with a diameter of 1.8 millimeters and a length of 60 millimeters designed using ANSYS SpaceClaim software, representative of typical micro-scale catalytic reactors used in laboratory studies and specialized applications.
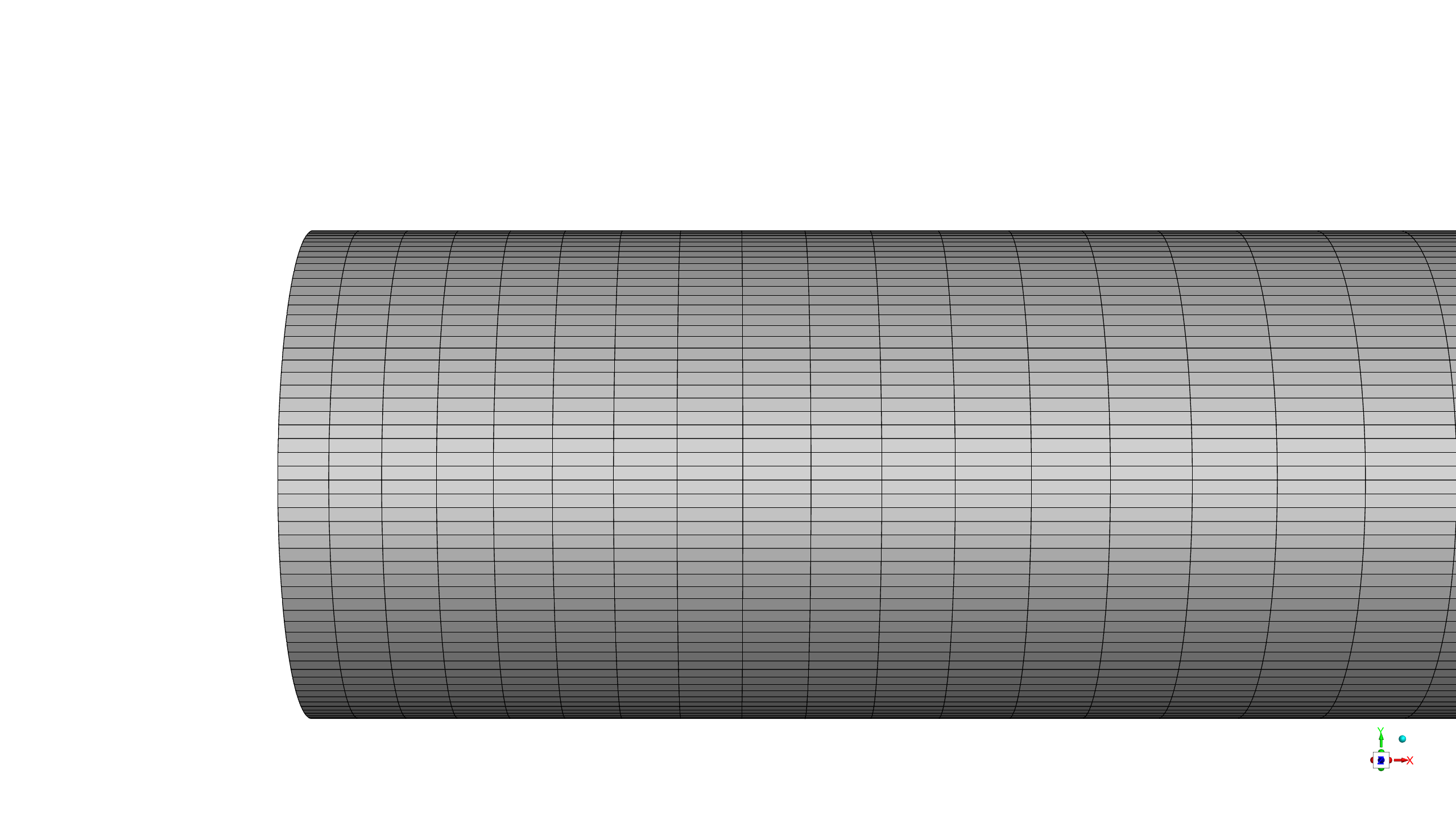
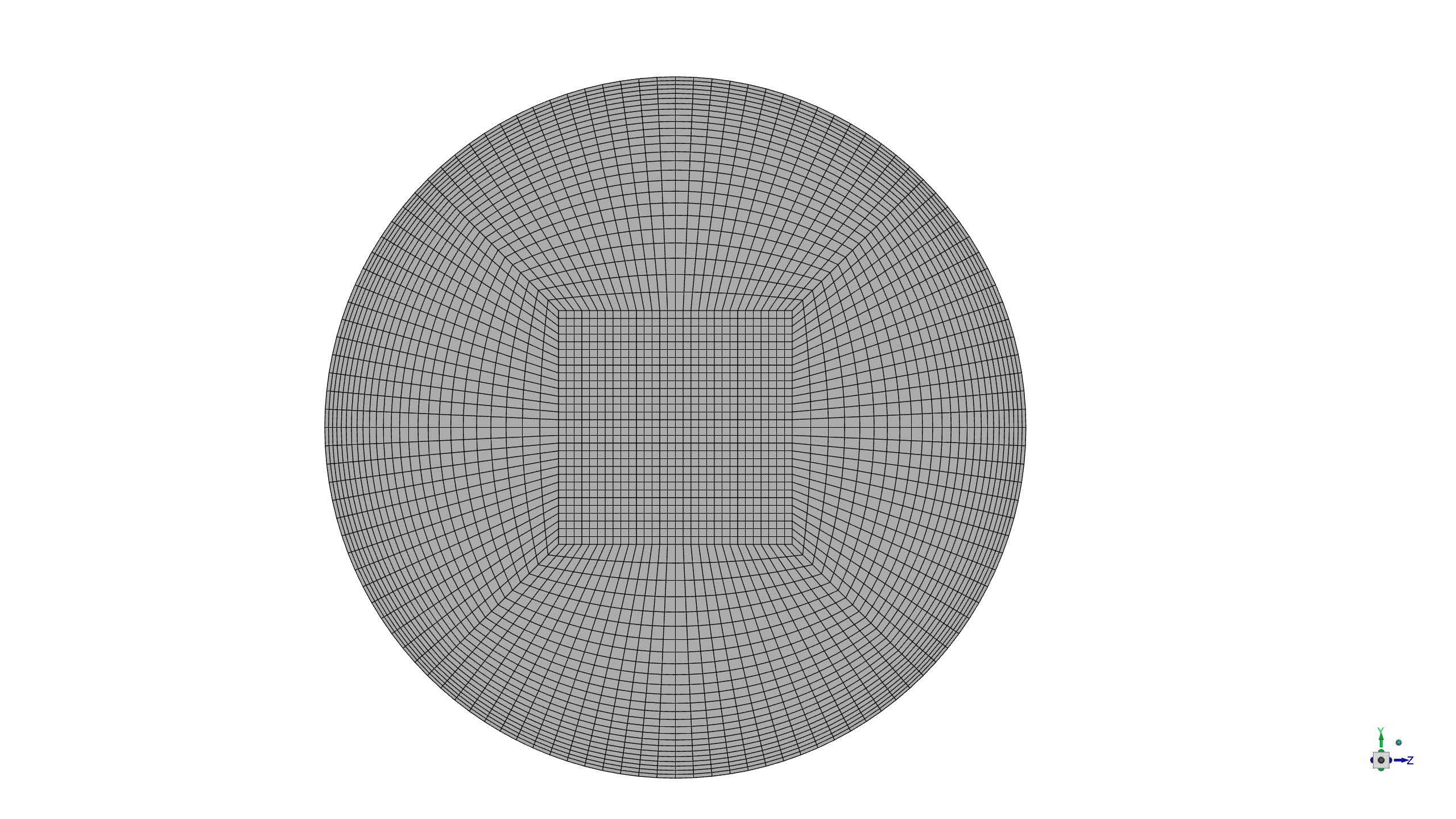
A high-fidelity computational mesh comprising 682,500 structured hexahedral elements was generated using ANSYS Meshing software to ensure accurate resolution of both the bulk flow phenomena and the critical near-wall region where surface reactions occur.
Methodology
The simulation employed a pressure-based, steady-state solver with a laminar flow model, appropriate for the low Reynolds number flow conditions. A comprehensive reaction scheme was implemented using the species transport model, incorporating both homogeneous gas-phase reactions for the volumetric combustion of methane and heterogeneous surface reactions on the platinum catalyst. The surface chemistry was modeled using detailed CHEMKIN mechanisms, which account for adsorption/desorption processes, surface diffusion, and catalytic reaction pathways.
Results and Conclusion
From the simulation, the following results are obtained:
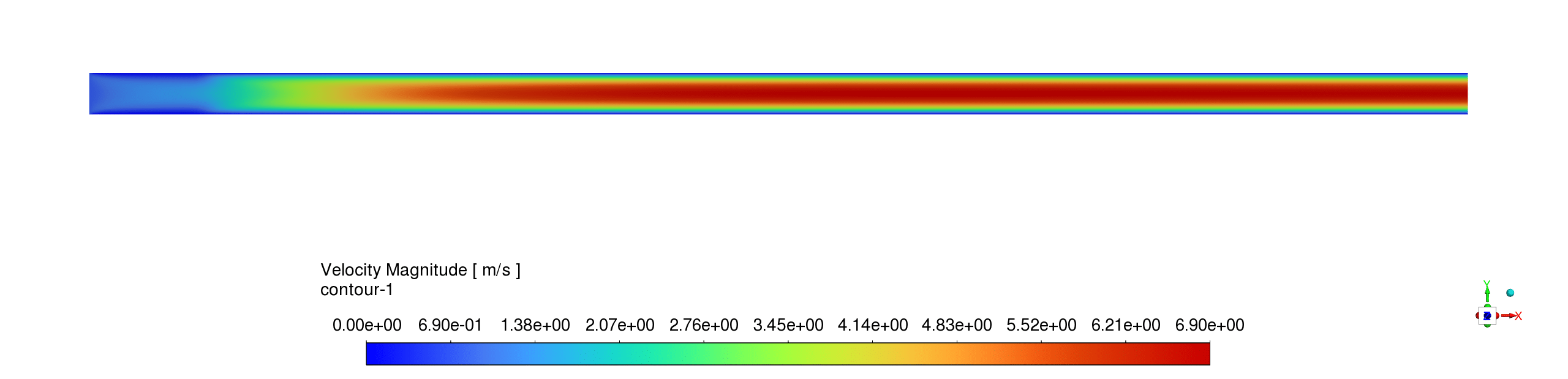
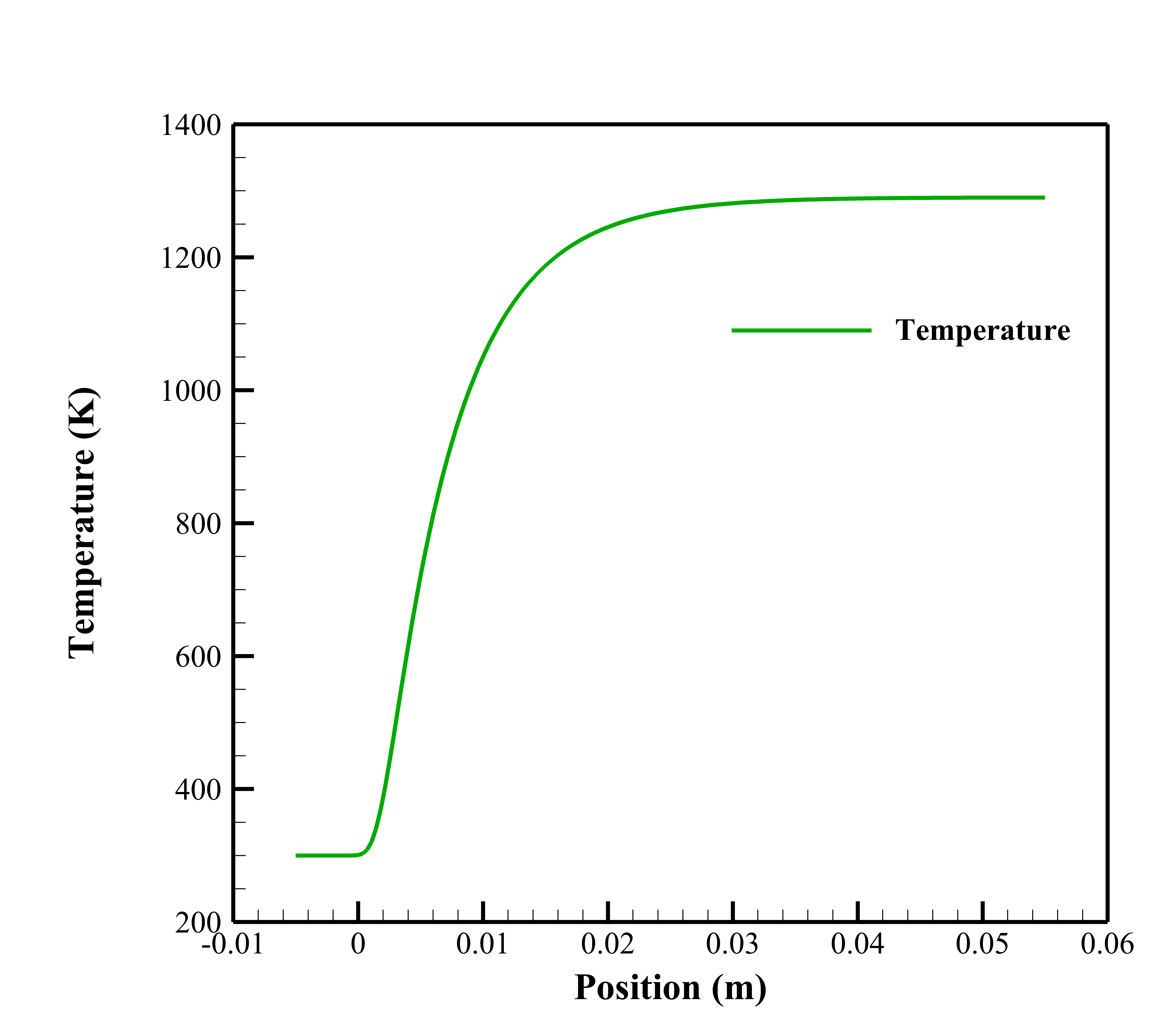
- The temperature distribution reveals a sharp reaction zone near the inlet where temperatures rapidly rise from ambient (~ 300 K) to peak combustion values (~ 1300 K), indicating an intense, localized exothermic reaction region that stabilizes approximately 5-10mm from the inlet before maintaining relatively uniform high temperatures throughout the remainder of the micro-pipe reactor.
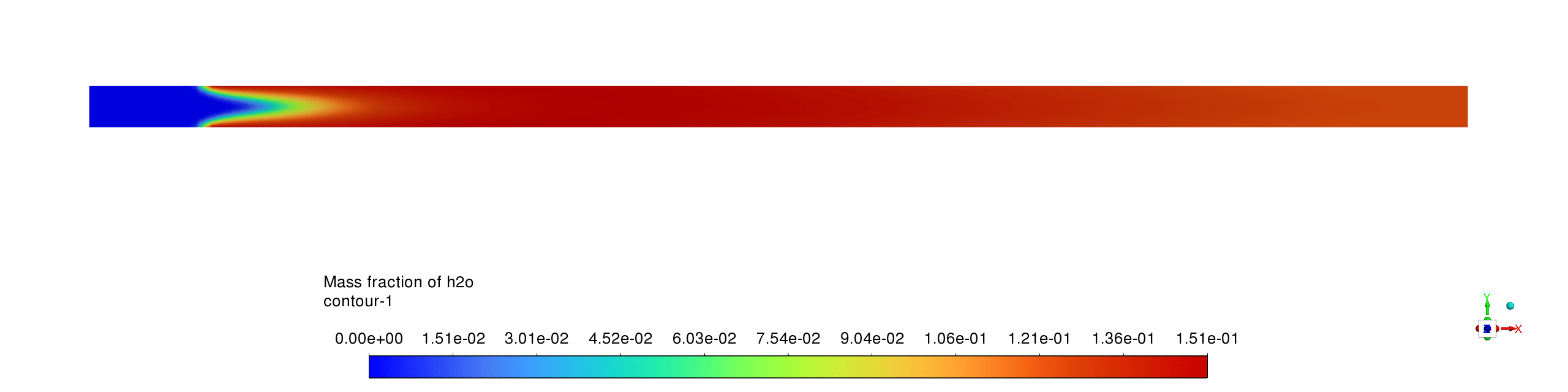
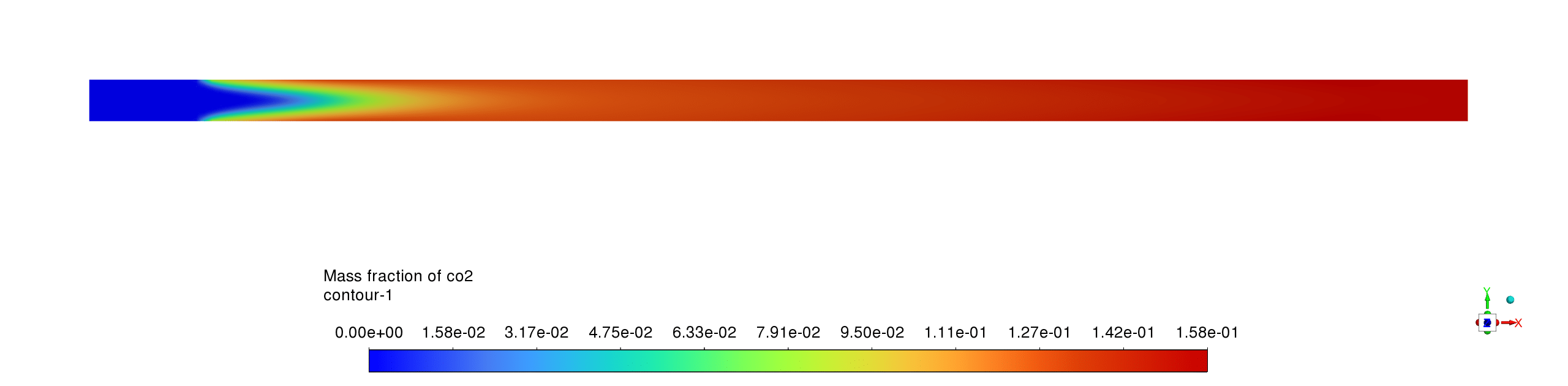
- The water vapor concentration profile shows a distinct formation zone coinciding with the reaction front, rapidly increasing from zero to approximately 0.15 mass fraction as methane is consumed, demonstrating the expected production pattern of a primary combustion product that remains stable downstream of the reaction zone.
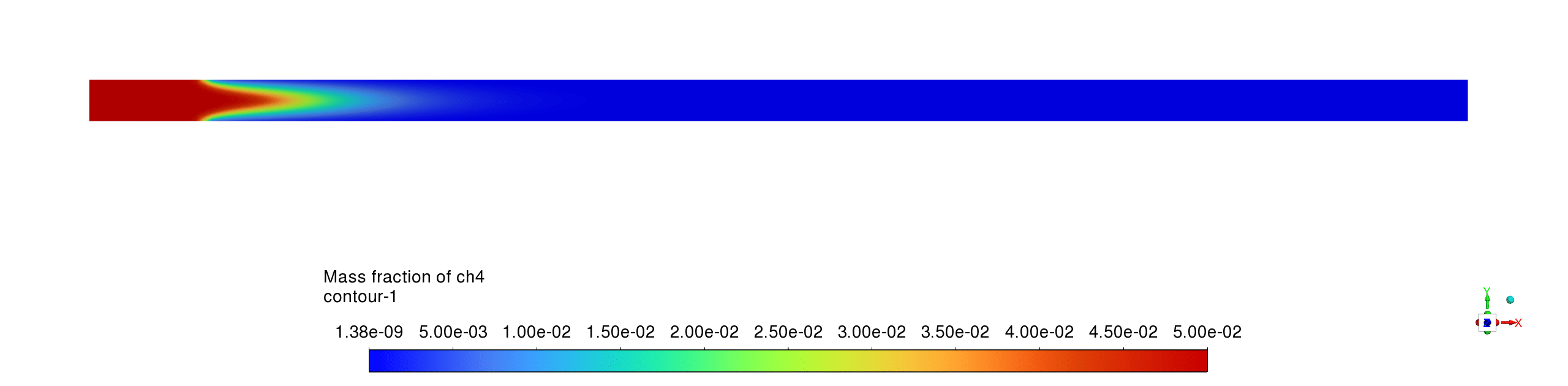
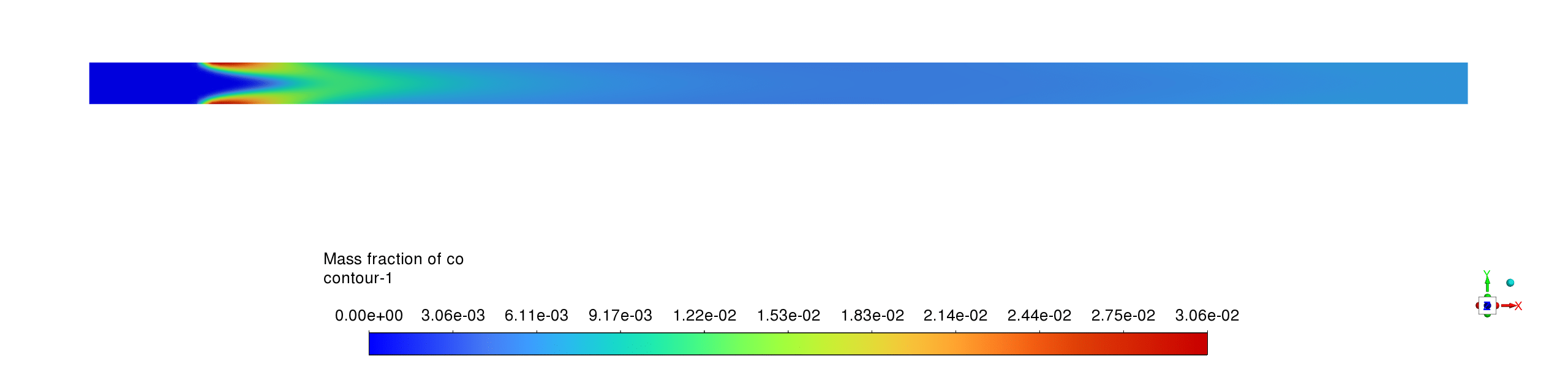
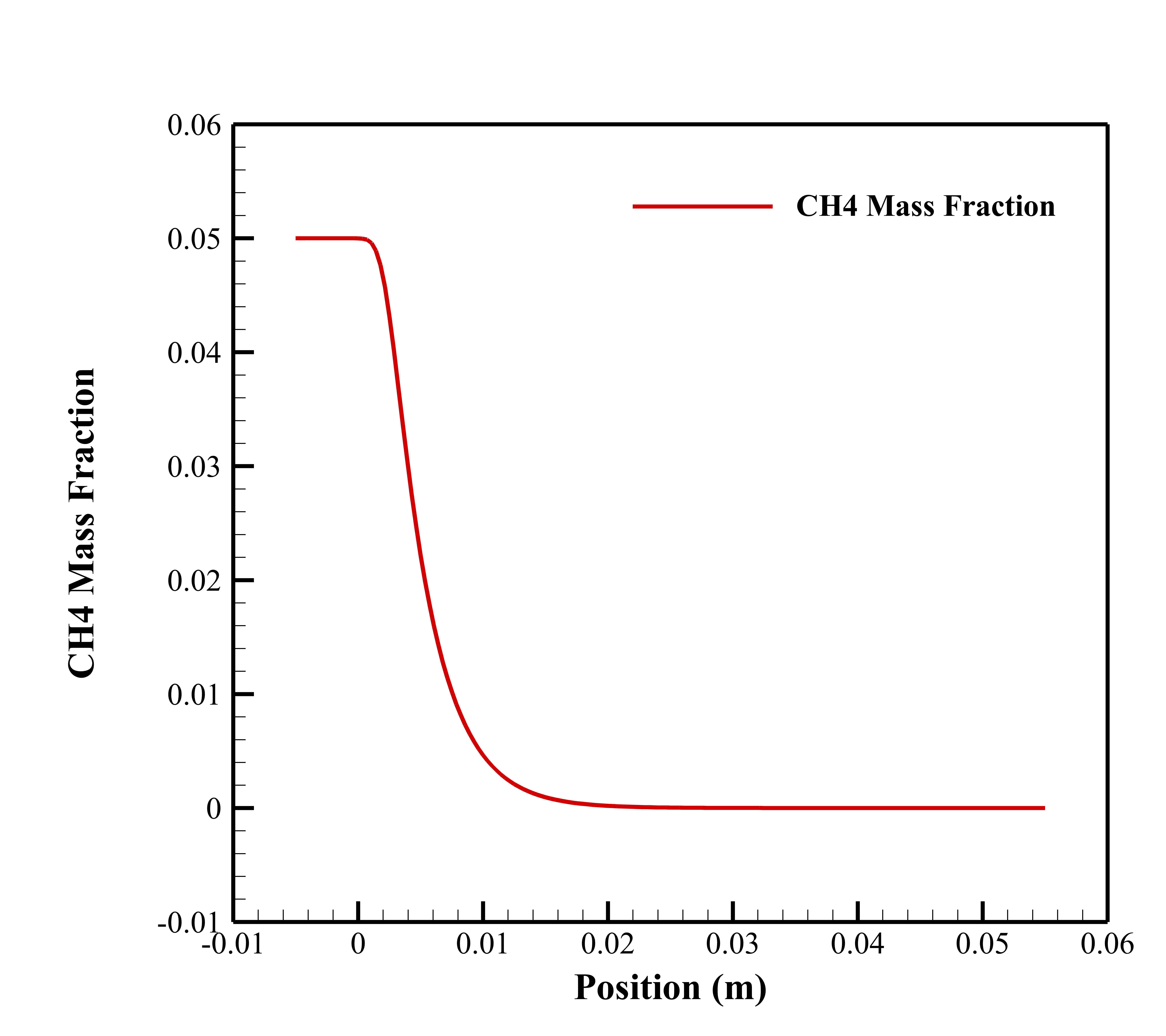
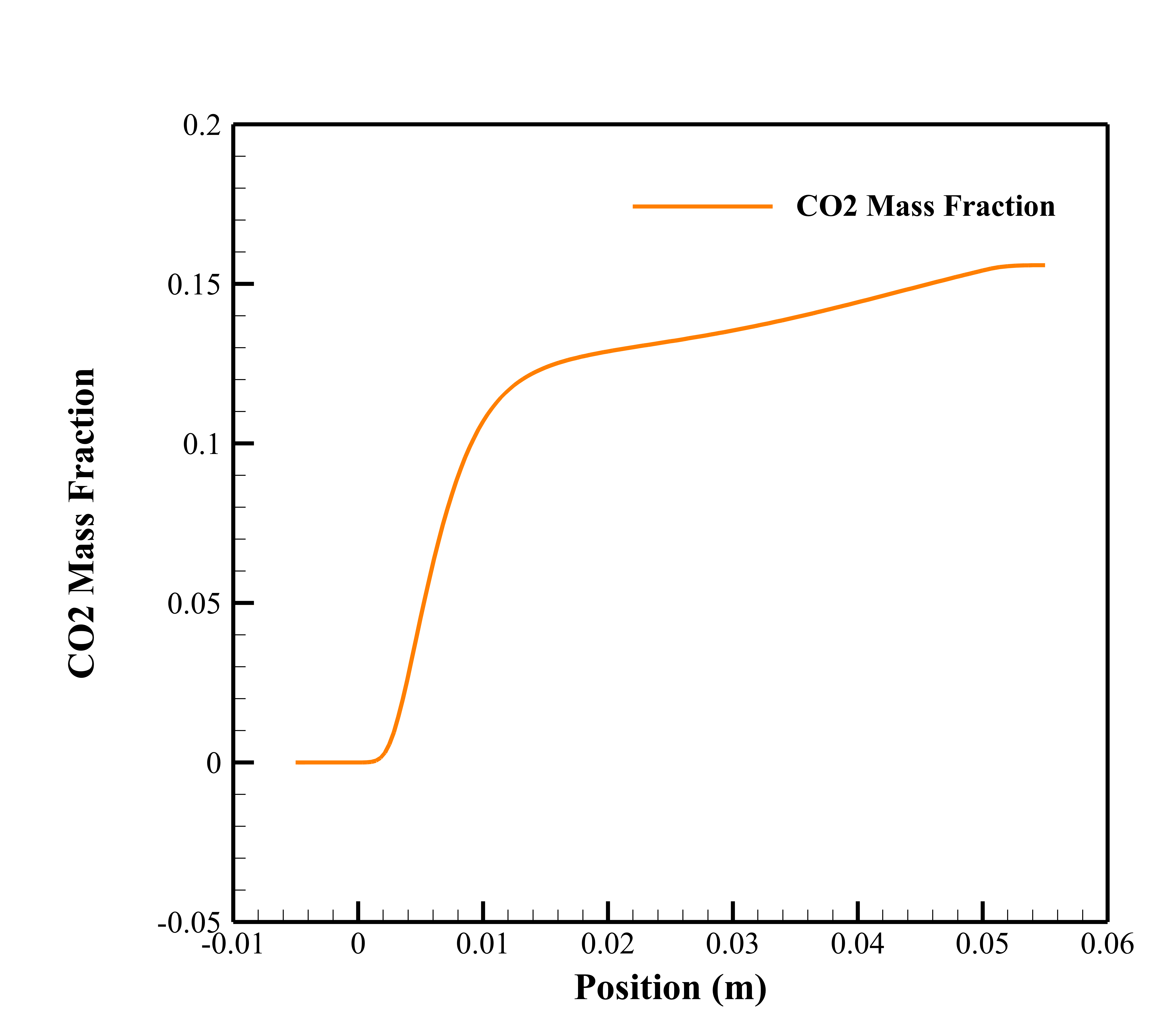
- The mass fraction contours reveal complementary reaction pathways with CH4 being rapidly consumed near the inlet while CO appears as an intermediate species before being partially converted to CO2; H2O shows the highest product concentration, confirming complete oxidation processes with the reaction sequence CH4→CO→CO2 occurring within a compact reaction zone, leaving negligible methane concentrations downstream and relatively stable product distributions throughout the remainder of the reactor.
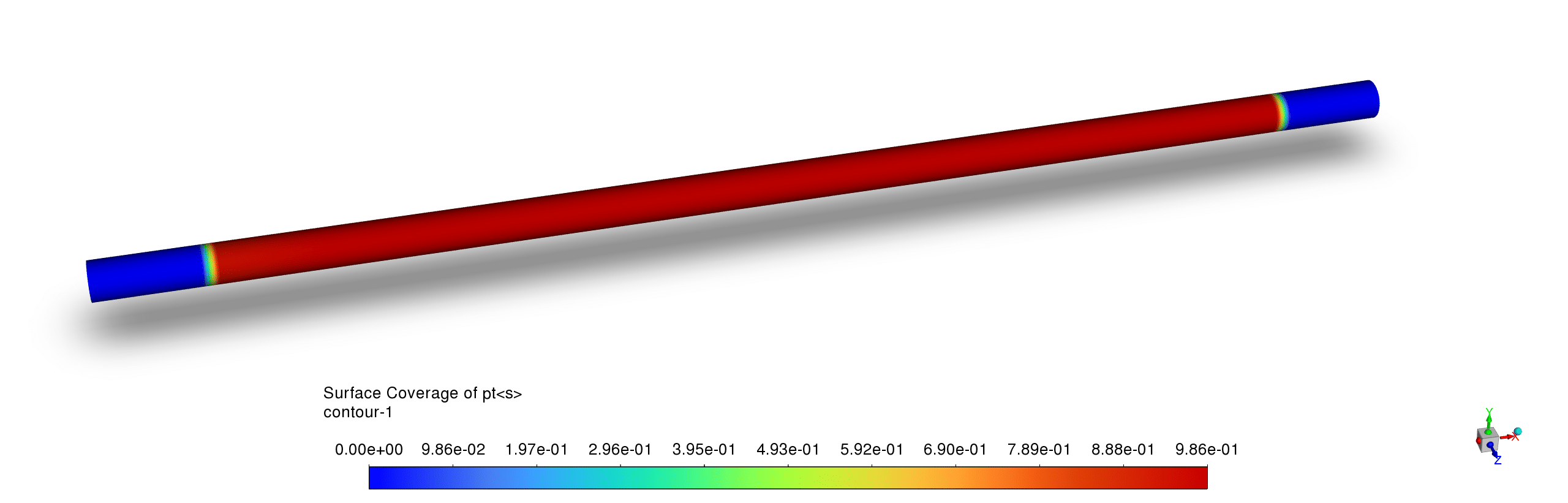
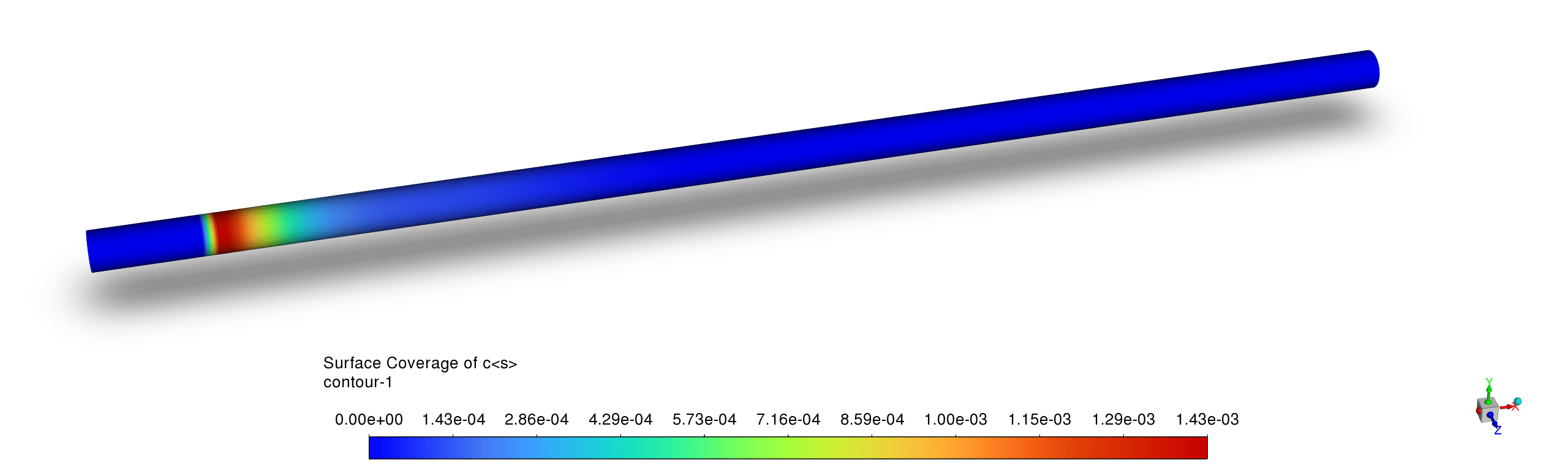
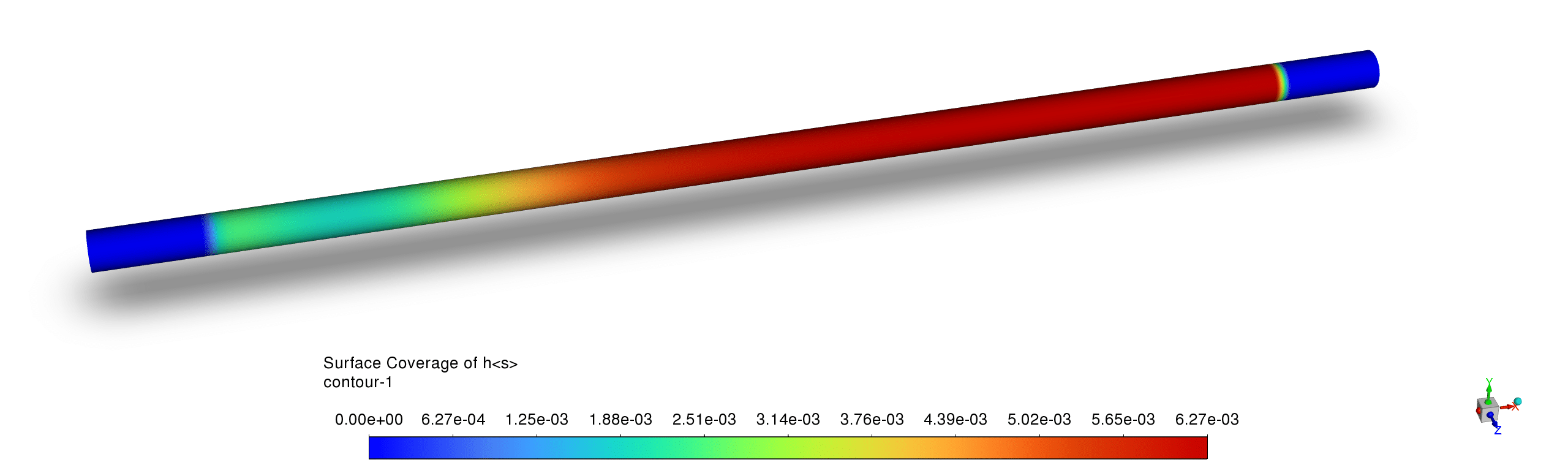
- The platinum surface coverage contours demonstrate a distinct catalytic activity pattern with high Pt site availability downstream of the reaction zone but significant occupation in the active reaction region, while H(s) and C(s) exhibit complementary coverage; this indicates that carbon and hydrogen species temporarily occupy active sites during the catalytic process but are efficiently consumed in the reaction pathway, preventing long-term catalyst poisoning.





Reviews
There are no reviews yet.